Chemistry Facts
Chemistry ['kĕ-mə-strē]
The study of the substances of which matter is composed.

When you hear the word “chemistry,” you may think of a scientist working in a lab, pouring liquids into tubes. But did you know that chemistry is all around you every day, and that it impacts many aspects of your daily life? Chemistry affects how your body digests food, how your water is made safe to drink, how your winter coat keeps you dry, how your computer's battery functions, how your rubber ball bounces, and how your favorite t-shirt gets its blue or red color. In everyday life, you're doing something that involves chemistry when you cook, when you take medicine, when you wipe off the counter with a cleaning detergent, when you apply sunscreen, or when you paint a picture. Chemistry is everywhere! Let's learn more about the science of chemistry.
Matter

Everything that has weight or mass and takes up space is called matter. Matter is the “stuff” in our world. Reach out and touch the nearest piece of furniture. That is matter. So are the clothes you are wearing, the hair on your head, and the air you are breathing. Everything that you can touch, see, feel, and smell — everything on Earth and even in the universe beyond — is made of matter.
Chemistry is the study of matter. When we learn about matter, we are learning about chemistry. Chemistry explores and explains the ways that matter changes, combines, and transforms to create the world we know. Since matter is all around us, chemistry is part of almost everything we do.
Atoms and Molecules
All matter is made of small particles known as atoms. Atoms are the basic building blocks of matter; they make up the air, the water, our bodies, food, trees, cars, and even the computer on which you are viewing this website. Atoms are so small that you cannot see them. But in the period at the end of this sentence, there are billions of atoms.
Atoms are made of three subatomic particles known as protons, neutrons, and electrons. The protons and the neutrons huddle together to make the nucleus in the center of the atom. The electrons orbit around the nucleus. Protons have what is called a positive (+) charge, while electrons have a negative (-) charge. Neutrons are not charged, but they affect the mass of the atom.
There are different types of atoms, and atoms combine in special ways to make up all of the matter in our world. That is why cat fur is so different from the steel bumper on a car. But both are still made of atoms. Atoms are different in the number of protons, neutrons, and electrons they contain.

When atoms join together, they form molecules. Molecules may be made up of the same kind of atom, or they may be made up of two or more different atoms. Although there are only 118 types of atoms, there are millions of types of molecules because the atoms combine in different ways.
States of Matter
Matter can exist on earth in different states or phases. The three states of matter you are most familiar with are solid, liquid, and gas. In solids, the atoms are packed tightly together and do not move past each other. Solids hold their shape at regular room temperature. With liquids, there is more space between atoms. Liquids do not hold their shape at room temperature, but instead take on the shape of the portion of the container they occupy – a glass, a pitcher, or the banks of a lake. Gases have so much room between atoms that they move freely and rapidly, and they spread out to fill all the available space in a container.



You can easily observe water in all three of these states. Water is a solid when it is ice, a liquid when we drink it, and a gas when it is steam. How does matter move from one state to another? If the motion of the atoms is altered by pressure or temperature, the state can change too. The atoms and molecules do not change, but the way they move about does. By lowering the temperature of water, it can freeze into ice which is a solid. By heating water, it can become steam which is a gas. Deep underground, extreme pressure causes solids to become liquid magma. Other substances need conditions produced in a laboratory setting in order to change states. But no matter which state, the molecular structure of the substance remains the same. Whether solid, liquid, or gas, water is still water.
To learn more, take a look at these animations of the behavior of solids, liquids and gases.
Physical Properties

A chemical is a substance that has specific properties that we can use to identify it. Atoms of a substance help give it the physical properties that we use to describe an object. Some physical properties of matter are color, odor, texture, hardness, and magnetism. If a substance allows heat or electricity to flow through it, the property is referred to as conductivity, while the ability to dissolve in a liquid is called solubility. Density, another physical property, describes how tightly atoms are packed together. An object with a higher density will have greater mass than a low-density object of the same size. The boiling point, the temperature at which a substance changes from liquid to gas, and melting point, the temperature at which a substance changes from solid to liquid, are also physical properties.
Chemical Properties

Atoms also give an object its chemical properties, but chemical properties cannot be determined just by looking at or touching an object. Chemical properties are identified by an object's ability to change given the right conditions. The ability of an object to ignite, for example, is a chemical property. The ability of an object to rust or tarnish is a chemical property, as is the toxicity of a substance, or its ability to damage a living organism.
Physical Changes

Matter can change in different ways. A physical change happens when we crumple up a piece of paper. It is still paper, just a different shape. We could change the temperature of a cup of water, mix two different paint colors together, cut a banana into slices, whip cream into peaks, or drop a glass jar and see it shatter into pieces. Despite all these different changes, the substances are still water, paint, banana, cream, or glass. Changes in size, shape, or states of matter are all physical changes.
Physical changes only affect the way that matter looks or feels. A physical change does not affect the chemical makeup of the object.
Chemical Changes
Chemical changes happen when changes are made to matter at the molecular level. Bonds between atoms are broken and new kinds of molecules are created. A chemical change creates a new substance that wasn't there before. When a chemical change happens, it is possible that you will see a change in color or odor, or you may find that heat, light, bubbles, fizzing, flames, or gases are given off. When you strike a match, for example, there is a sound of fizzing, there is heat, and there is a strong smell as gases are given off. These are signs that a chemical change has happened. You've actually created a new substance.

Chemical changes happen all around us. When a piece of fruit ripens, that is a chemical change. You cannot change the fruit back into its un-ripened state. When you put bleach in the washing machine to clean your clothes, a chemical change breaks down molecules in the stains. When wood burns in a campfire and produces smoke and ash, the atoms have been rearranged into new combinations, and the properties of the starting materials have changed.

Sometimes a chemical change happens in a slow reaction, as when rust is produced. Rust happens over a long period of time when iron is exposed to oxygen. But rust is a new chemical. It isn't iron and it isn't oxygen; it is rust.
The process by which substances are changed into other substances is called a chemical reaction. Chemical reactions are part of everyday life. When you eat, your body uses chemical reactions to break down food into energy. Photosynthesis in green plants, where plant food and oxygen are produced from water, sunlight, and carbon dioxide, is a chemical reaction.
Elements
Everything in the universe is made from elements. Elements make up the basic ingredients of all matter. We know of 94 elements that occur in nature, but several more have been created by scientists for a total of 118. An element is a pure substance that is made from one single type of atom. For example, gold is an element that is made up of gold atoms – it cannot be broken down into smaller components and still be called gold. Oxygen is an element made up of oxygen atoms. Copper is an element made up of copper atoms.
The most common and abundant elements make up most of the matter on Earth. Although your body is made of trillions of atoms, just four elements – hydrogen, carbon, oxygen, and nitrogen – account for more than 96% of those atoms inside you. And as far as we know, elements make up matter throughout the universe. Iron atoms found on Earth are identical to iron atoms found in meteorites and in the red soil of Mars.

The Periodic Table of Elements is used to organize the elements by their atomic number, or the number of protons in the nucleus. For example, oxygen has 8 protons, so its atomic number is 8. Chemical symbols stand for each element. There is much more you can learn about atoms and elements from the periodic table.
Compounds and Mixtures
Although a few things in our world are made of just one element, such as oxygen, carbon, or gold, most are a combination of two or more elements. While there are only 118 elements, there are millions of combinations of elements, making different types of molecules. When different elements are chemically bound together, they are known as compounds. When a compound is created from different kinds of atoms, the new substance created does not have the same physical or chemical traits of the original elements. Each compound has its own unique properties.
Compounds are written with formulas showing which elements from the periodic table are combined. One very familiar compound is water. Water is made of two elements chemically bound together. When two hydrogen atoms (H2) combine with one oxygen atom (O), it makes the compound H2O, which we know as water. The formula H2O tells us that there are 2 atoms of hydrogen bound to each atom of oxygen. All water molecules have this same combination of atoms. Water is not hydrogen or oxygen. You couldn't pour oxygen and hydrogen atoms on a fire and expect to put it out. But when they are bonded together as water molecules, they behave like water.
The same elements in different amounts can build very different compounds. If you took those two hydrogen atoms and joined them to two oxygen atoms (instead of one), you would wind up not with water but with H2O2, a very different compound called hydrogen peroxide – and you wouldn't want to drink it!

There are many other compounds that are already familiar to you. When one sodium atom (Na) combines with one chlorine atom (Cl), it makes the compound NaCl, which you know as salt. Every time you breathe out, your breath contains CO2, a compound of one carbon atom (C) and two oxygen atoms (O2) that we call carbon dioxide. Sometimes more than two elements make up a compound. A sugar molecule (glucose) is a compound of 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms, written as C6H12O6. These specific atoms in these exact numbers make up a sugar molecule.
Sometimes substances may combine without forming a compound. A compound requires a chemical reaction where bonds are formed between atoms and a new substance is created. Without that chemical reaction, combined substances may instead form a mixture. The components of a mixture keep their original properties and can easily be separated. For example, a mixture of fruits in a salad can be separated back into groups of different fruits. Salt and water can be combined in a mixture, but water is still water, and salt is still salt. To separate the two components, the water can be evaporated so that the salt can be collected. Sand and water can be separated by using a filter. The ocean, rocks, blood, and even the air we breathe are mixtures rather than compounds.
Acids and Bases

Chemists often describe liquid chemicals as acids or bases. Acids have a sour taste. Some acids, such as lemon juice and vinegar, are foods we can eat. Our stomachs use acids to help digest foods and to kill bacteria that could make us sick. Other kinds of acids are used in car batteries to make them run. Those strong acids are dangerous and should never be handled by students.

Bases are the opposite of acids. They have a bitter taste. Soap is a common base found around the house. If you have ever accidentally gotten shampoo in your mouth while washing your hair, you have experienced its bitter taste. Other common bases are baking soda and toothpaste. Very strong bases can be dangerous and can harm living cells.
Sometimes people use an antacid to treat their upset stomach. An antacid is a base which, when dissolved in the stomach's acid, neutralizes that acid and takes away its acidic qualities. The acid no longer bothers the person's stomach and they feel better. That is chemistry at work in our lives.
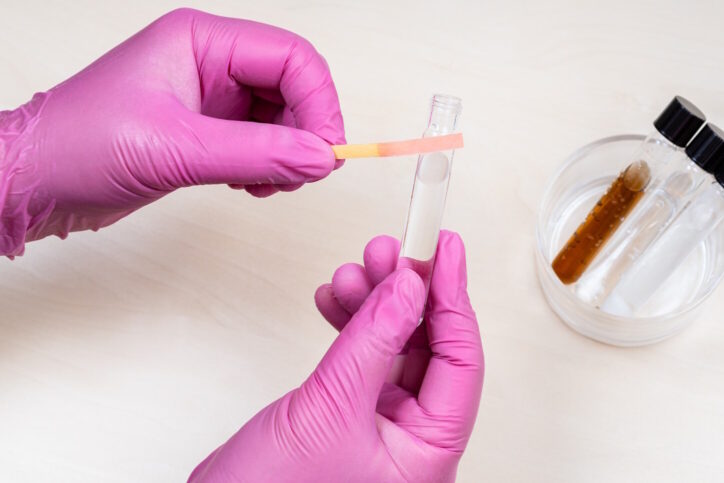
To determine if a substance is an acid or a base, chemists can test it using special paper known as litmus paper which changes color when exposed to chemicals. This is known as testing the pH of a substance. By comparing the color of the changed paper, chemists can tell whether the substance is an acid or a base. Chemists use a scale between 0 and 14 to describe how strong the acid or base is. Pure water is 7, right in the middle, and most everyday liquids are in the middle range. Very strong acids (with a pH near zero) and very strong bases (with a pH near 14) are dangerous and should be handled only by people who are trained to work with them. Chemicals should never be tasted without adult supervision.
Chemistry and You
Chemistry really is everywhere! Everything you hear, see, smell, taste, and touch involves chemistry and matter. Chemical reactions power all life on Earth and keep our bodies functioning. Chemistry allows scientists to create medicines that keep us well. Chemistry is responsible for turning used milk jugs into carpets for homes and schools, and for creating sunscreen that keeps us from getting sunburned. Chemistry has been used to enrich soil for growing crops, to measure levels of pollution in the environment, and to make stronger metals, fibers, and building materials. Chemistry allows scientists to determine what kinds of matter make up the planets and stars, even from millions of miles away. Nearly everything you see around you at home is some type of chemical compound, from vinegar to nail polish remover to baking soda to aspirin. Chemistry has even come up with fun products such as glow sticks, silly string, and marshmallows!

Scientists are learning more and more about chemistry all the time. The work of chemists is to research all aspects of matter and its properties and changes, and then create new substances that improve people's lives. Sometimes such substances are discovered by accident, like the glue used in Post-it notepads and the fabric protectant known as Scotchgard. Both chemicals were invented in chemical laboratories, but they were discovered as an accidental application of the chemist's work.
Learn more at Science Trek's websites for Compounds and States of Matter. Keep learning all you can about chemistry. Perhaps you will be the future chemist who finds a way to power cars using the air we breathe!
Fun Links
Visit the American Chemical Society's website for kids, Adventures in Chemistry. You'll find experiments, games, activities, and fascinating facts about chemistry in everyday “stuff.” Create an explosion, dissolve some M&Ms, and make your own slime!
The Jefferson Lab offers a full range of chemistry-related activities for you. Check out “All About Atoms” at Homework Helpers, explore the interactive Periodic Table of Elements, and design atoms along with the video tutorial at How To Draw An Atom. Then have fun with the Element Games and Puzzles — crossword puzzles, matching games, concentration, and more.
Explore kitchen chemistry with Ruff Ruffman and Scruff from PBS Kids, as they compete in a cook-off while learning about the properties of matter.
Learn more about all aspects of matter, atoms, elements, and reactions at Chemistry For Kids.
Join Sam, Mia, RJ, and Zoe in these Study Jams videos: Atoms, Elements & Compounds, Acids & Bases, Periodic Table, and Physical & Chemical Changes. Test yourself by taking the quiz after each video.
Science Buddies has a great collection of chemistry activities and science projects for you to do at home with a parent's help.
At DK Find Out's interactive site, colorful photos, and diagrams help explain matter, elements, compounds, atoms, molecules, atomic particles, and chemical reactions.
Find out more about the history and basic principles of chemistry at Brittanica Kids and Kiddle Encyclopedia.
Science Kids: Chemistry has some fun videos, experiments, quizzes, and activities for you to check out.
At SmartClass4Kids, illustrated, colorful pages help you understand more about chemistry topics. Check out physical properties of matter, chemical and physical changes, and elements and compounds.
Here are some fun chemistry videos for you to watch:
- What's Matter? (Crash Course Kids)
- Chemical Changes (Crash Course Kids)
- What Is Chemistry? (Science Classroom)
- Chemistry & Chemists (SciShow Kids)
- Chemical Reactions (SciShow Kids)
From the American Museum of Natural History, make a mobile of a carbon atom and learn more about the periodic table.
Top 10 Questions
March 2017
Thanks to Dr. Kathryn Devine, associate professor of physics at the College of Idaho and Dr. Christopher Saunders, chemistry lecturer at Boise State University, for the answers.
-
What exactly is chemistry?
The simple answer is that chemistry is the study of matter, and matter is the stuff that makes up everything that you see and feel around you. You could say that chemistry is the study of everything and how everything in the universe interacts with each other. (From Tyler at McDonald Elementary School in Moscow)
-
What are atoms?
Atoms are the fundamental building blocks of matter. If you take matter and look at the tiniest things that it is made of, you will find atoms. For example, hydrogen is an atom. Oxygen is an atom. If you take two hydrogen atoms and an oxygen atom and put them together, you will have a molecule of water. Everything around us is made from molecules and atoms. When put together, these tiny particles build our world. (From Halie at Mountain View Elementary School in Boise)
-
Why are some chemicals poisonous to people and others are not poisonous?
That is a complicated question. Even things that you may not think of as being poisonous or toxic to people, can be toxic in the right dosages. In determining if something is harmful to someone, it is important to know how much of the substance that person has had exposure to. (From Ashley at Russell Elementary School in Moscow)
-
How did scientists decide which elements are on the periodic table, and in what order they are?
The person who is credited with the construction of the periodic table is Dmitri Mendeleev. He organized the elements based on the number of protons that you find in the nucleus. All of the elements that we know are on the periodic table and, we continue to add new elements. The order and the way that the periodic table is organized tells us something about the chemical properties of those elements. (From Amelia at McDonald Elementary School in Moscow)
-
How is baking chemistry?
When you bake, you rely on chemical reactions to get certain results, just like you do with chemistry. One of the most common examples is the use of baking soda or baking powder. Although there are differences between the two, both of them rely on acid-based chemistry. For example, when you use baking soda in a cake mix, the baking soda will dissolve with moisture as it heats. It will then interact with an acid, and that reaction produces carbon dioxide, causing the mixture to rise, becoming what we know of as cake. (From Emerson at McDonald Elementary School in Moscow)
-
How does liquid nitrogen and dry ice stay so cold?
Anything that is at an extreme temperature or a different temperature from the area around it, when left out in the open, it will reach thermodynamic equilibrium. This means that it will eventually become the same temperature as the room around it. For dry ice and liquid nitrogen to stay cold, you have to prevent that from happening. To do this, you need to put them in something insulated. (From Cora at Russell Elementary School in Moscow)
-
Why are Newton's three laws so important?
Newton's three laws describe forces in motion. These laws allow physics to be described in terms of rules and lay the groundwork for how we teach modern physics. (From Scott at Dalton Elementary School in Dalton Gardens)
-
What does H2O stand for?
H2O is an example of a chemical formula. The way that scientists write chemical formulas tells you what the composition of the molecule is. With H2O, the "H" stands for Hydrogen, the "O" stands for Oxygen, and the "2" is referencing how many atoms of hydrogen there are per oxygen. We call H2O, water. Water has two atoms of hydrogen and one atom of oxygen. (From Louie at Riverside Elementary School in Boise)
-
How did people discover electrons and neutrons?
The person who is credited with the discovery of electrons is J. J. Thompson. In his experiments, he discovered that if you looked at different materials and exposed them to electricity (you put a lot of energy into them), they would produce a beam of rays. We call them cathode rays. All of these rays are negatively charged. If you exposed them to something that is positively charged, the beam of particles would be attracted. Later on, the term electron started being used for those particles. Thompson won the Nobel Prize in 1906 for this discovery. (From Ella at Christine Donnell School in Boise)
-
How do light bulbs work, and how are light bulbs now more efficient?
There are the "old fashioned" light bulbs that have the filament inside. A filament is a wire. When you put it into a light bulb and screw that into a socket, a current runs through it. This heats the filament up and it starts to emit light. The light is in the form of visible light that we can see, and in the form of infrared light that we can feel as heat. If you were to put your hand near one of the older bulbs, you would feel the heat that it emits. Newer light bulbs have a CFL, or compact fluorescent light bulb. These light bulbs have a gas inside of them. When you screw one of these bulbs into a socket, the current actually excites the electrons, creating a higher energy level in the gas inside the light bulb. Then, as those electrons cascade back down, they give off photons. The photons hit the coating on the inside of the light bulb, which causes a phosphorescence of fluorescent reaction. That gives off the light we can see with our eyes. The reason these bulbs are more efficient is that they do not emit as much heat. In other words, they give off energy but more in the form of light than heat. (From Caleb at Riverside Elementary School in Boise)
